The controversy of adult hippocampal neurogenesis in humans: suggesting a resolution and way forward
Neurogenesis is more than just a concept in neuroscience, and has nuances that go beyond mere rattling statistics. It is the Catch-22 of the world of learning and development in humans and possibly other animals. Modestly rendered as a phenomenon of de novo neuronal birth in the brain, neurogenesis represents a process that imbibes utmost significance from developmental point of view and maintenance of normal brain morphology and neurological functioning. This process may very well be a sort of brain housekeeping realm. Neurogenesis is believed, and aptly so, to be the vital basis of learning in animals, including humans. It is pertinent to note that song birds (zebra finches) learn new tunes every season, rodents explore almost everything during their entire life and tend to solve intricate problems with their sense of smell and progressive learning. And who would ignore kids, who are always curious, and have amazing learning abilities? Human kids provide amazing examples of learning and curiosity that has given us the obstetric dilemma.
In the animal brain, neurogenesis primarily happens in the hippocampus, which is the part of the brain involved in formation and consolidation of memories and learning of new skills. The last four decades of work on animal models have amassed cumulative evidence of continued hippocampal neurogenesis during the adult age. To be more precise, we are here talking about dentate gyrus. These results have found reasons for extrapolation to adult humans to account for the unavailability of direct evidence. (This is, of course, not Edward Jenner's time!) Crystalizing all the evidence for neurogenesis in a single sentence, it would be safe to say that we are sure about neurogenesis in the developing human brain. That raises the question: Can we use a similar expression for neurogenesis in adult human brains?
Continued hippocampal neurogenesis is an imperative requisite in perpetually learning biological systems, and therefore, would be an obvious need in adult humans for learning new skills and accommodating experiences for an integrated neurocognitive whole. The induction of adult hippocampal neurogenesis (AHN) has been shown to improve spatial learning and memory in transgenic mouse models in addition to resilience against neuropsychiatric disorders associated with a decline of pertinent cognitive functions.
Going down the track of history, adult brain neurogenesis was first reported in adult mice (by Altman and Das in 1965) and then in song birds (by Goldman and Nottebohm, in 1983). Following these two reports, hundreds of studies appeared in the literature piecemeal, completing the picture and weeding off the wool. Some of these studies included those on humans. Gradual accumulation of literature bolstered the notion that adult neurogenesis could be a reality in humans.
However, in 2018, Sorrells et al from University of California, San Francisco, presented some surprisingly contrary evidence in the journal Nature, demonstrating sharp decline in neurogenesis in children and complete absence of this process after 13th year of age in humans. This report unsettled the somewhat established opinion of AHN. Many neuroscientists identified relatable flaws in the methodology employed by Sorrells et al, and some even presented fresh pieces of evidence in order to re-establish the old (and rather optimistic) idea. However, the ripples of destabilization of AHN were such that it led to a controversy that could not easily be resolved.
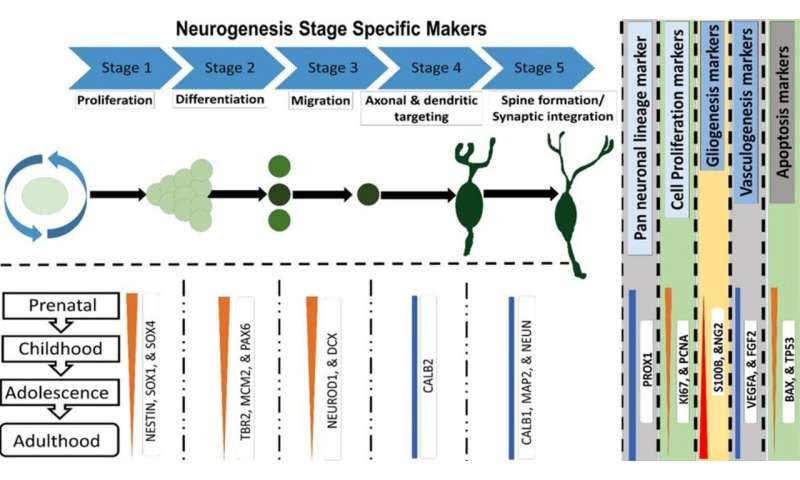
Our group, working under the umbrella name of Etiologically Elusive Disorders Research Network (EEDRN), recognized the resolution of this controversy as a worthwhile aim. This ardent task could be materialized by analyzing signature neurogenesis genes, including genes for gliogenesis, vasculogenesis and apoptosis (programmed cell death), in post-mortem human hippocampal tissues from fetal to adult age. We studied expression of these genes in the context of developmental stages; a total of five developmental stages are described for hippocampal neurogenesis as shown in Fig. 1. In order to fulfill the objectives of for this purpose, we used developmental transcriptome data from the Allen Human Brain Atlas.
We found a borderline situation in the adult human hippocampus, which could surprise both supporters and skeptics of AHN in humans. It seems to further complicate the issue, but in reality, it paves the way for the resolution of the ongoing tussle and hence shows path forward. Teleologically, one would expect the markers for all neurogenesis genes to show significant expression across all age groups in case of continued neurogenesis. Also, if AHN is residual a process, neurogenesis-associated genes would be expressed with a downward trend, and with a parallel upregulation in the expression of apoptotic marker genes as the age advances.
To our surprise, our analysis revealed that the genes expressed by neural stem cells and progenitors were downregulated over time; remarkably, the expression of cell proliferation markers (KI67 and TBR2) was almost negligible by adolescence. However, there was no parallel reduction in the total number of immature neurons and early mature neurons. We also found a perpetual downregulation of apoptosis markers, albeit without detectable significant differences between successive age groups. Additionally, we failed to find any significant age-related reductions in the expression of the key vasculogenesis genes, which serve as proxy markers for neurogenesis.
Based on the transcriptomic signature we identified, a linear progression of neurogenesis (and as well as gliogenesis) from progenitor cell pools looks like an obvious conclusion. Progression of neurogenesis is restricted after childhood, and reduces to negligible levels around adolescence and onwards. As we observed a fairly maintained expression of genes for immature and mature neurons until adult age, we zeroed in on an alternative possibility that partial and limited neurogenesis may continue in adolescents and adults from a developmentally arrested pool of immature neurons. This notion is bolstered by some recent studies as well.
Having made an attempt to put the debate to rest and having provided an explanation of contrasting reports, our findings have been published in the current issue of the open-access journal IBRO Reports, a publication of the International Brain Research Organization (IBRO).
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information: Ashutosh Kumar et al. Transcriptomic analysis of the signature of neurogenesis in human hippocampus suggests restricted progenitor cell progression post-childhood, IBRO Reports (2020). DOI: 10.1016/j.ibror.2020.08.003
Kumar et al. Adult neurogenesis in humans: A review of basic concepts, history, current research, and clinical implications. Innovations in Clinical Neuroscience (2019). www.ncbi.nlm.nih.gov/pmc/articles/PMC6659986/
Contributors/ Author Affiliations: An international team of neuroscientists from various reputed institutions including All India Institute of Medical Sciences (AIIMS), Patna, National Brain Research Centre (NBRC), Manesar, and Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, and New York University (NYU) School of Medicine, New York, Medical University of South Carolina, Charleston, U.S., and Aix Marseille University, Inserm, France.
Bio:
Dr. Ashutosh Kumar is an assistant professor in Department of Anatomy, All India Institute of Medical Sciences (AIIMS)-Patna, Bihar, India.
Dr. Vikas Pareek is a doctoral affiliate from National Brain Research Center (NBRC), Manesar, Haryana, India.
Dr. Muneeb A. Faiq is a post-doctoral affiliate from New York University (NYU) Langone Health Center, NYU Robert I Grossman School of Medicine, New York, New York, USA.